Hepatitis C Genotype 2 Treatment
 |
HCV Genotype2A and 2BTreatment and Cost
|
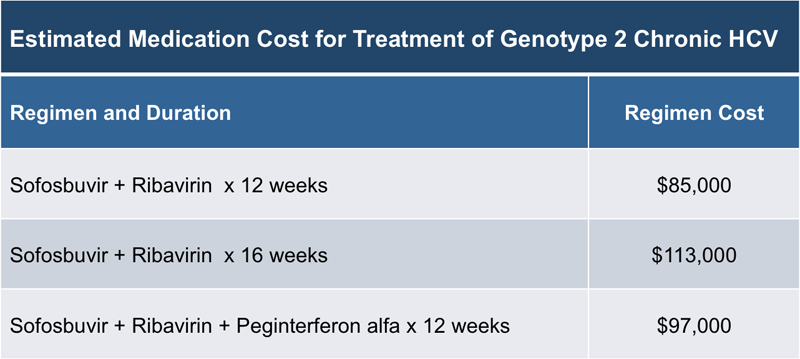
In the United States, genotype 2 accounts for approximately 13 to 15% of all hepatitis C infections. Given the historically relatively high sustained virologic response (SVR) rates with the treatment of genotype 2, the data regarding retreatment of patients with genotype 2 in whom prior therapy failed is somewhat limited. The following discussion regarding initial treatment and retreatment of patients with genotype 2 chronic hepatitis C assumes the patient and their clinician have already made the decision to proceed with hepatitis C therapy. The FDA approval of the newest, highly effective, well-tolerated direct-acting antiviral agents (DAAs) has been complicated by the high price of these new agents. For the regimens included as preferred or alternative in the 2014 AASLD/IDSA/IAS-USA Guidance for genotype 2 infection, the cost of the treatment regimens range from approximately $85,000 to $113,000 (Figure 1). Although company-related drug assistance programs provides free medication to some low-income patients, getting medications paid for remains problematic for many clinicians and patients.
Historically, treatment of genotype 2 infection achieved higher sustained virologic response (SVR) rates than with genotype 1 infection, even with a shorter duration of therapy and lower doses of ribavirin. Until recently, the standard of care for treatment-naive patients with genotype 2 hepatitis C has consisted of a 24-week course of peginterferon plus fixed-dose ribavirin, with SVR rates of 75 to 85%. In 2013, the FDA approved a 12-week course with the all-oral regimen of sofosbuvir plus ribavirin for the treatment of genotype 2 infection based on data from several studies showing SVR rates of approximately 95% with this regimen. The approval of this regimen represented a landmark introduction of interferon-free therapy for chronic hepatitis C. No hepatitis C protease inhibitors have received FDA approval for the treatment of genotype 2 HCV, but simeprevir has shown in vitro activity against HCV genotype 2.
Factors to Consider Prior to Choosing Treatment Regimen: For patients chronically infected with genotype 2 hepatitis C, two major factors determine the optimal treatment regimen and duration: (1) whether the patient has previously received and failed therapy and (2) the presence or absence of cirrhosis. Hepatitis C therapy in patients with decompensated cirrhosis, renal impairment,
Click Here for More information on Hepatitis C Genotype 2 Treatment
Source: Hepatitis C Online