Epigenetics of drug abuse: predisposition or response
Overview
Originally Published: 05/22/2015
Post Date: 05/22/2015
by David A Nielsen, Amol Utrankar, Jennifer A Reyes,, Daniel D Simons,, and Thomas R Kosten
Summary/Abstract
Drug addiction continues to be a serious medical and social problem. Vulnerability to develop an addiction to drugs is dependent on genetic, environmental, social and biological factors. In particular, the interactions of environmental and genetic factors indicate the significance of epigenetic mechanisms, which have been found to occur in response to illicit drug use or as underlying factors in chronic substance abuse and relapse. Epigenetics is defined as the heritable and possibly reversible modifications in gene expression that do not involve alterations in the DNA sequence. This review discusses the various types of epigenetic modifications and their relevance to drug addiction to elucidate whether epigenetics is a predisposing factor, or a response to, developing an addiction to drugs of abuse.
Content
Drug addiction, a chronic relapsing brain disease, is a major medical and social problem. Approximately 22.1 million people in the USA are classified as demonstrating substance dependence or abuse [101]. Over 1 million people are addicted to cocaine and over 350,000 to heroin. From 2002 to 2010 the number of people addicted to cocaine decreased from 1.5 to 1.0 million, while those addicted to heroin increased from 214,000 to 359,000, and the number of prescription opiate abusers rose to over 1 million. Currently, 17.9 million people are alcoholics, in comparison with 18.1 million in 2002 [102].
Addiction develops in several stages: initiation of drug use, intermittent to regular use, and finally, addiction and relapse. Features of addiction are the development of dependence on the drug, such that there is a physiological need for the drug for the individual to function properly; the development of tolerance, whereby larger doses of the drug are required to achieve the same effect; and the development of withdrawal, symptoms that occur once a drug is discontinued. Drugs of abuse alter physiological systems, contribute to the maintenance of the addictive state and influence withdrawal and relapse [1,2].
Vulnerability to addiction and chronic addiction are influenced by convergent biological, social, environmental and genetic factors [3]. Twin studies have revealed that there are common heritable genetic components that predispose an individual to drug addiction, and that these genetic factors contribute approximately 20–50% to the variance of developing a drug addiction, with the remaining contribution due to nongenetic factors [4–6]. Recent studies have elucidated the inter-related nature of these determinants, clarifying the idea that individual biological factors and broader biosocial influences interact.
Recent studies have revealed the role of gene–environment interactions, which occur when genetic factors interact with the environment to influence behavioral phenotype. For example, several studies have focused on the 5-HTTLPR variation in the promoter of the serotonin transporter SLC6A4 gene, which codes for the serotonin transporter, a target for cocaine and 3,4-methylenedioxy-N-methylamphetamine (ecstasy) [7]. The short 5-HTTLPR allele decreases both serotonin transporter expression and serotonin uptake [8]. For example, in a study of adolescents, the rate of substance abuse initiation in subjects with one or two copies of the short allele was moderated by exposure to supportive parenting or membership in community-building initiatives [9]. Studies such as this demonstrate that environmental conditions can regulate, or fully attenuate, genetic predispositions to psychiatric conditions such as addiction vulnerability.
Although the effects of gene–environment interactions remain unclear, there may be more of a genetic influence on exhibited phenotypes than traditional nature–nurture dichotomy studies would indicate. In the context of drug addiction, the interactions between genotype and environmental factors point toward an important role for epigenetic mechanisms in the acute response to drugs and the development of addiction. This epigenetic perspective is consistent with the longevity of psychiatric conditions and the difficulty in developing pharmacotherapeutic interventions to effectively treat chronic behavioral disorders.
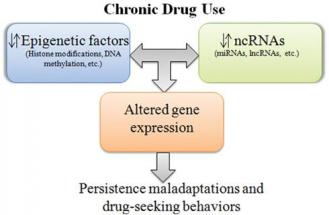
Epigenetics is the regulation of the heritable and potentially reversible changes in gene expression that occur without alterations in the DNA sequence [10]. The primary mechanisms controlling epigenetic inheritance are DNA methylation and chromatin remodeling. Epigenetic modifications can be immediate or accumulate slowly, and may be passed on to daughter cells or to successive generations through mitotic or meiotic inheritance. These epigenetic alterations may be due to inheritance through genomic imprinting, prior life events, chronic drug use or pharmacotherapies for the addictions.
This review discusses the role of epigenetics in response to drugs of abuse, as well as the epigenetic changes observed in drug addiction and withdrawal. One question that this article aims to elucidate is whether epigenetic alterations increase vulnerability to develop a drug addiction, or whether addiction itself constitutes an epigenetic response to these drugs. This discussion is limited to cocaine, opioids and alcohol (Table 1), and will minimally review the basic concepts of epigenetics.
Summary of epigenetic modifications in drug abuse.
Basic concepts of epigenetics
One of the major epigenetic pathways modifying gene expression is the methylation of cytosines at the 5′ position of the cytosine pyrimidine ring in CpG dinucleotide sites. DNA methylation is critical for proper organismal development, genetic imprinting, X-chromosome inactivation and tissue-specific gene expression [11–14]. In general, methylation of CpG sites in promoter regions leads to decreased gene expression and has evolved as a method for tagging genes for silencing.
The first methyl-CpG-binding protein identified was MeCP2, and this protein is relevant to addictions [15,16]. Knockdown of Mecp2 in the nucleus accumbens, a brain region involved in reward in adult mice, enhanced amphetamine-induced conditioned place preference, whereas Mecp2 overexpression reduced conditioned place preference [16]. This suggests that MeCP2 in the nucleus accumbens limits the rewarding properties of psychostimulants. Psychostimulants drove dopamine-dependent phosphorylation of the amino acid Ser421 on MeCP2, a site regulating MeCP2 repressor function, revealing a mechanism whereby drugs regulated MeCP2 function. This increase in phosphorylation of MeCP2 in the nucleus accumbens was found to be correlated with increased behavioral sensitization. Another study found that in extendedaccess rats, but not restricted-access rats, knockdown of MeCP2 in the dorsal striatum increased cocaine intake [17]. MeCP2 inhibited the expression of miR-212, and miR-212 inhibited Mecp2 expression. The dorsolateral striatum has been shown to mediate the rewarding properties of cocaine reinforcement, while the nucleus accumbens shell may be involved in the motivational aspects of cocaine reinforcement [18]. The role of MeCP2 in these two brain regions may differ.
Changes in DNA methylation may contribute to memory consolidation in the hippocampus and storage in the cortex, both of which are critical processes in addiction [19]. The role of key enzymes in DNA methylation, such as DNA methyltransferases, in regulating the induction of synaptic plasticity in the hippocampus, is relevant to the neuroplasticity changes observed in drug addiction. CpG methylation changes may be stable or revert rapidly. For example, in the hippocampus of rats exposed to fear conditioning, the Pp1 gene had a 120-fold increase in DNA methylation after 1 h, which returned to baseline in 24 h [20]. Conversely, the differentially methylated region of the imprinted IGF2 gene was found to be hypomethylated in subjects exposed prenatally to famine during the Dutch Hunger Winter of 1944–1945, indicative of events that occurred 60 years ago [21].
Chromatin is composed of DNA and proteins. Modifications to chromatin structure in the immediate vicinity of a gene determine the extent to which that gene is expressed [22]. The fundamental unit of chromatin is the nucleosome, comprised of an octomer that contains two copies of each of the four histones: H2A, H2B, H3 and H4, around which DNA is wrapped. Histone cores are tightly packed with the aminoterminal tails of each histone exposed and subject to modifications. The modifications found on each histone tail form specific combinations that establish whether chromatin is in an activating (‘open’) or repressive (‘closed’) transcription state. Acetylation, methylation and phosphorylation of these tails can be points of transcriptional regulation. Histone acetylation has been shown to be associated with an open chromatin structure that promotes transcriptional activity [23] and may occur through the inhibition of histone deacetylases or the activation of histone acetyltransferases [24]. Similarly, histone phosphorylation is associated with transcriptional activation [25]. By contrast, histone methylation, controlled by histone methyltransferases and histone demethylases, is quite complex [26]. For example, some histone methylations, such as H3K27me3, are found on histones in transcriptionally inactive chromatin, while other methylations (e.g., H3K4me3) are on histones of active chromatin. Other forms of histone modifications, such as ubiquitylation, sumoylation and poly-ADP-ribosylation are beginning to emerge as additional regulators of transcription [27].
DNA methylation and chromatin modifications are linked processes. DNA methylation may be a signal to package DNA in a closed chromatin conformation. In mammalian cells, MeCP2 is recruited to the Sin3A-histone deacetylase corepressor complex, which can deacetylate histones and signal the formation of repressive chromatin structure [28]. Conversely, DNMT3A can interact with the amino-terminal tail of H3 and methylate linker DNA regions [29]. This binding is disrupted by di- or tri-methylation of lysine 4 (K4) of histone H3.
The modulation of gene expression that mediates short- and long-term drug-induced neural plasticity may be a result of histone modifications that have been found to play a role in learning and memory [19,30,31]. The learned association between environmental stimuli and drug administration, and the processes that are involved in the formation and recollection of this association, are vital in the maintenance of self-administration and in relapse [32–34]. Covalent histone tail modifications may also be partially responsible for the continued and persistent changes that drugs of abuse can have on gene expression following withdrawal. These gene-expression changes may influence the susceptibility to relapse, playing a role in ‘cellular memory’ [35].
Both acute and chronic administration of drugs of abuse alter gene expression in a number of regions in the brain [36,37]. Manipulation of the epigenetic state of specific genes involved in drug-induced neuronal plasticity may offer a pharmacotherapeutic approach to the treatment of drug craving and relapse. A greater understanding of the essential role of epigenetic modifications in regulating behavioral responses to drug exposure may help in the discovery of new pharmacotherapies for drug addiction.
Genomic imprinting is the phenomenon in which the expression of a gene is dependent upon the parent of origin for that gene. Imprinting is most commonly studied in the context of gene silencing, which results from an epigenetic modification, presumably both DNA methylation and histone alterations, in the parental gamete cells that inhibits one of the inherited alleles in the offspring [38,39]. Such differential marking of genes, based on parent of origin, can yield a range of developmental and biological variation, and behavioral adaptations that influence the offspring for the duration of its lifespan.
Epigenetic alterations caused by drugs of abuse & addiction
Epigenetic changes in DNA methylation and chromatin remodeling have been found to occur in response to illicit drug use. The epigenetic state of chromatin may be an underlying factor in the development of chronic substance abuse. Administration of a drug of abuse triggers epigenetic alterations that regulate transcription. These changes in gene expression may influence reward, psychomotor activity, drug craving and relapse. An individual’s vulnerability to develop drug addiction, their response to drugs of abuse or their response to pharmacotherapy for the addictions may be determined, in part, by epigenetic factors such as DNA methylation and histone modifications.
Cocaine
Behavioral addiction and neuronal plasticity, in part, are rooted in epigenetic mechanisms. Chromatin remodeling has been implicated as a factor in long-term development of cocaine addiction. Accumulation of the transcription factor subunit ΔlFosB, a truncated splice variant of FosB, in the striatum of the brain is characteristic of the administration of several stimulants, narcotics and depressants, and reflects gene activation. Administration of cocaine, in both acute and chronic studies, has produced both immediate and lasting gene-expression changes through epigenetic modifications. In rodent studies, both c-fos (also referred to as cFos or Fos) and ΔFosB expression is activated by cocaine [40,41]. In the nucleus accumbens, a single acute cocaine injection rapidly increases immediate early gene expression, including expression of c-fos, indicating a role for c-fos in mediating the initial response to psychostimulants [42]. Acute and chronic cocaine has been shown to cause hypomethylation of the FosB promoter, decreased MeCP2 binding and upregulated FosB expression in the nucleus accumbens [43].
Acute cocaine has been shown to cause chromatin remodeling at the c-fos promoter, and chronic cocaine to cause chromatin remodeling at the FosB promoter in the mouse striatum [44]. Acute cocaine administration has been shown to induce H4 acetylation and increased gene expression at the c-fos promoter in the mouse striatum, a modification that was not observed with chronic cocaine administration [44]. This c-fos epigenetic alteration was reversed after chronic exposure to cocaine. The acetylation of H4 at the FosB promoter was through recruitment of the CREB-binding protein to the FosB promoter [45]. This histone H4 acetylation upregulates ΔFosB and FosB expression, with an associated increase in cocaine sensitivity. A gradual build up of ΔFosB attenuates c-fos activity by recruiting HDAC1 to the c-fos promoter, which inhibits c-fos expression and desensitizes c-fos activity following repeated cocaine administration [46]. Long-term elevated levels of ΔFosB also cause a gradual accumulative effect that intensifies the reward response and locomotor activity [47]. Chronic cocaine exposure decreases the activity of HDAC5 in the nucleus accumbens, thereby increasing transcription of HDAC5 target genes [48]. Treatment of the nucleus accumbens prior to cocaine administration with the histone deacetylase inhibitor, sodium butyrate, increases the reward sensitivity and locomotor activity effect of cocaine [44]. Chronic cocaine also induces the histone H3 acetylation of the CaMKIIa gene, which codes for Ca2+/CaMKII-α, and increases its expression [49]. These gene-expression changes support the hypothesis that drug addiction is an epigenetically regulated response, as the diminished levels of histone deacetylases and increased histone acetylation contribute to an increased reward in response to substances of abuse.
Acute cocaine induces Dnmt3a and Dnmt3b expression in the mouse nucleus accumbens and causes hypermethylation of the Pp1 promoter, increased MeCP2 binding and decreased Pp1 expression [43]. It has been demonstrated that within 20 min of injection, acute administration of cocaine causes phosphorylation of MeCP2 at serine 421 in the rat striatum and nucleus accumbens, thus preventing it from functioning as a transcriptional repressor [50]. Inhibition of MeCP2 repressor activity may then upregulate the expression of downstream genes that are critical to the response to chronic cocaine. MeCP2 phosphorylation in rats induces transcription of Bdnf and FosB, which have been shown to be upregulated in cocaine users, supporting the idea that MeCP2 phosphorylation is an essential initial process in the epigenetic response to psychostimulants [51]. Acute exposure to drugs of abuse appears to alter a specific set of epigenetic events that change with chronic exposure.
Exposure to one drug of abuse may have consequences on the effects of a different drug of abuse. In mice, nicotine pretreatment increased the behavioral response to cocaine and enhanced the depression of cocaine-induced synaptic potentiation [52]. FosB expression in the striatum was increased by 7, but not 1, days of nicotine treatment indicating a long-term neuroplastic change. Nicotine alone increased FosB expression, which was not attenuated by pre-exposure to cocaine. Long-term exposure to nicotine stimulated the acetylation of both histones H3 and H4 at the FosB promoter and reduced histone deacetylase activity. Furthermore, the histone deacetylase inhibitor suberoylanilide hydroxamine acid (SAHA) enhanced cocaine response in a similar manner to that of nicotine. Many cocaine addicts had initiated smoking prior to developing cocaine addiction. It may be that these epigenetic effects of nicotine may increase the vulnerability of nicotine users to become addicted to cocaine, in part, by heightening their sensitivity to cocaine.
In contrast to the downregulation of c-fos with chronic cocaine, Bdnf and Cdk5 are upregulated in mice after chronic cocaine administration via histone H3 acetylation in the striatum [44]. As ΔFosB accumulates in the nucleus accumbens, it induces the expression of Cdk5, a ΔFosB target. Treatment with a Cdk5 inhibitor or targeted Cdk5 knockout in the nucleus accumbens in chronically exposed animals has been associated with increased reward sensitivity and locomotor activity, implying that Cdk5 regulates dopamine neurotransmission and causes long-term desensitization in these brain areas of cocaine users [53,54]. Similarly, Bdnf contributes to the neuroplasticity found in chronic cocaine addiction by regulating self-administration and relapse behavior [55].
In the mouse hippocampus, expression of Bdnf has been shown to be unaffected by acute or chronic cocaine treatment, but increased following withdrawal [56]. However, in the nucleus accumbens shell Bdnf expression was increased by either acute or chronic cocaine administration and by withdrawal, indicating the brain region-specific effects of cocaine. While Cdk5 levels in the striatum started to normalize after a week of withdrawal, Bdnf levels remained elevated, and even continued to increase after withdrawal [57], implying a continual and long-lasting epigenetic response to chronic addiction at the Bdnf gene. Moreover, repeated injections of Bdnf into the nucleus accumbens increased the amount of effort that animals invested into receiving cocaine and potentiated the magnitude of relapse behavior [55]. The gene-expression patterns and epigenetic alterations at the Cdk5 and Bdnf gene promoters are reflective of the behavioral adaptations in long-term cocaine users. Cdk5-induced desensitization mirrors the tendency of human subjects to develop tolerance to a fixed-level dose of cocaine, which often motivates users to seek higher doses to counteract attenuation. The steady build up and long-lasting stability of these epigenetic mechanisms demonstrate the capability of chromatin remodeling to contribute to chronic addiction following repeated exposure to drugs of abuse. These two model systems of Bdnf and Cdk5 have reinforced our understanding of the epigenetics surrounding drug use as a neuroplastic response.
miRNAs are small RNA molecules that transregulate gene expression, degrading target mRNAs and, in some cases, repressing translation. miR-212 was reported to be upregulated in response to cocaine in the striatum of rats, leading to an amplification of the stimulatory effects of cocaine on CREB signaling [58]. miR-212 amplified CREB signaling, presumably through phosphorylation of Raf-1, leading to increased sensitization of adenylyl cyclase activity.
The transgenerational effects of cocaine have also been studied. Maternal cocaine exposure has been found to produce both behavioral and physiological alterations in the offspring. Maternal cocaine exposure in mice has demonstrated decreased global DNA methylation at P3 and increased global methylation at P30 in hippocampal pyramidal neurons [59]. Expression of DNMT1 and DMNT3a, but not DNMT3b, was increased at P30 but not P3. Paternal exposure to cocaine has also been shown to influence physical and behavioral deficiencies in pups, demonstrating a role for transgenerational effects originating in the fathers and transmitted through the germ cells [60]. These studies on the biological parent-to-offspring effects of cocaine administration are particularly important as they emphasize the transgenerational pathologies that can be inflicted from chronic cocaine use. Although the socioeconomic and psychological effects of parental cocaine use on offspring is extensively researched, inherited genetic and epigenetic effects are important for understanding the full hereditary impact of substance abuse.
Opioids
Central to the development of opioid addiction is the μ-opioid receptor, encoded by the OPRM1 gene, the target for the bioactive products of heroin (6-monoacetylmorphine and morphine), most opioid analgesic medications and the endogenous ligands, the enkephalins and β-endorphin [1]. In vitro studies have demonstrated that increased OPRM1 promoter methylation and histone deacetylation is found to be associated with decreased OPRM1 mRNA content [61,62]. Activation of OPRM1 occurs during differentiation of P19 cells when the transcription factor Sp1 and the chromatin remodeling factors Brg1 and BAF155 bind to the OPRM1 promoter [63]. Concurrently, dissociation of histone deacetylase and the repressors mSin3a, Brm and MeCP2 was observed. Recruitment of Brg1 may promote the dissociation of MeCP2 with concomitant histone modification to activate OPRM1 expression [64]. Inhibition of histone deacetylase function with sodium butyrate enhances morphine-induced conditioned place preference and locomotion sensitization with no effect on morphine dependence or tolerance [65].
DNA methylation at the OPRM1 promoter was found to be different between former heroin addicts stabilized in methadone maintenance than in controls [66]. Two specific CpG sites in the promoter region of the OPRM1 gene have been found to be hypermethylated in DNA from peripheral lymphocytes of Caucasian former addicts. Both sites are located in potential Sp1 transcription factor-binding sites. A subsequent study found differences in DNA methylation of the OPRM1 promoter between former heroin addicts and controls among different ethnicities [67]. The degree of methylation was lower in African–American addicts than controls at a single CpG site, and higher in Hispanic addicts at three CpG sites, when compared with controls. Another recent study reported hypermethylation at seven OPRM1 CpG sites in leukocyte DNA of male opioid addicts compared with controls [68]. In addition, they found hypermethylation at one CpG site in the OPRM1 promoter in sperm, suggesting the heritability of an epigenetic change resulting from opioid addiction.
Given that DNA methylation of the OPRM1 promoter reduces transcription and thereby reduces levels of the μ-opioid receptor, a negative feedback mechanism may cause the hypomethylation found in the OPRM1 promoter region in Caucasian and Hispanic former heroin addicts to attenuate OPRM1 expression and thus reduce μ-opioid receptor levels. This is supported by the report of reduced μ- as well as δ-opioid receptors in the blood of methadone maintenance subjects [69]. It is possible that hypermethylation of CpG sites in the OPRM1 promoter may block the binding of Sp1, as well as other transcription factors, leading to OPRM1 silencing.
Transgenerational effects of opioid exposure have been demonstrated in rats. Female adult offspring of dams who were treated with morphine for 10 days prior to mating had more anxiety-like behavior than those of control dams, and the male offspring of treated dams had increased sensitivity to the analgesic effects of morphine and developed tolerance to chronic morphine more rapidly [70]. Hence, epigenetic effects of prior exposure to opioids of dams may be passed on to the next generation, altering morphine sensitivity and anxiety-like behavior, although maternal behavior or prenatal environmental differences cannot be excluded.
Alcohol
Alcohol produces epigenetic changes after both acute and long-term use. Chronic ethanol treatment has been shown to cause demethylation of CpG islands in the NMDA receptor NR2B subunit gene NR2B in the cortical neurons of mice and an increase in NR2B expression [71]. Chronic intermittent ethanol treatment and subsequent withdrawal was found to increase NR2B gene expression with a concomitant increase in H3K9 acetylation of the NR2B promoter region in primary cortical neurons [72]. This increase was not due to a change in global histone acetyltransferases or histone deacetylase activity, but probably a decrease of G9a, Suv39 h1 and HDAC1–3 binding to the NR2B promoter. Acute ethanol administration has also been shown to decrease histone deacetylase activity and to increase H3 and H4 acetylation in the amygdala of rats [73]. Conversely, withdrawal after chronic ethanol treatment produced an increase in histone deacetylase activity in the same regions, with a concurrent decrease in H3 and H4 acetylation. These alterations in histone acetylation during withdrawal were associated with increased anxiety in rats undergoing withdrawal as compared with rats treated with trichostatin A (TSA), an inhibitor of histone deacetylase activity, in which anxiety-like symptoms did not develop. The anxiety associated with ethanol withdrawal may be attributable, in part, to changes in histone acetylation, suggesting that alcohol withdrawal symptoms may be attenuated using histone deacetylase inhibitors.
Incubation of rat hepatocytes with ethanol for 1 day has been shown to increase acetylation at H3K9 in a dose-dependent manner [74]. Intragastric administration of ethanol to rats over a 1-month period was also found to increase H3K9 acetylation. This increase was due to an increase in p300 histone acetyltranferase activity, which is known to bind to CREB-binding protein, another histone acetyltransferase [75]. The increase in p300 histone acetyltranferase activity may have been triggered by liver hypoxia via two possible mechanisms, an increase in the transcription factors HIF-1α-β or through a decrease in SirT1 deacetylation of histones caused by a decrease in NAD, a requisite SirT1 coenzyme.
The role of dynorphin in the treatment of alcoholism is of interest since the opioid receptor antagonist naltrexone decreases alcohol craving. Treatment of human neuroblastoma cells with acetaldehyde, the initial metabolite of ethanol, for 2–3 days downregulated prodynorphin PDYN gene expression. This was accompanied by increased H3K27 trimethylation, and decreased H3K4 trimethylation and H3K9 acetylation at the PDYN promoter region [76]. Administration of ethanol for 3 days caused an increase in H3K9 acetylation. In post-mortem studies, prodynorphin gene expression was found to be higher in the dorsolateral prefrontal cortex, a region involved in cognitive control, of alcoholics compared with controls [77]. In this region, elevated methylation of three CpGs in the 3′-UTR of PDYN was found in DNA from alcoholics compared with controls, indicating increased expression when methylated. One of these CpGs was created by a genetic (C>T) variant. The C allele is protective against developing alcoholism [78], but in alcoholics with this allele, it was found to be hypermethylated, perhaps modifying the binding site of a transcriptional regulator.
Hypermethylation has been found in the DNA of the lymphocytes of alcoholics when compared with controls. Global DNA methylation in lymphocytes was increased in alcoholics, although DNMT3b expression was reduced [79]. Hypermethylation of the HERP gene has been found in alcoholics and was associated with elevated levels of homocysteine, which enhances expression of endoplasmic reticulum chaperone proteins, which may protect the endoplasmic reticulum from stress [80,81]. Other genes in which DNA hypermethylation has been found to be associated with alcoholism include the α-synuclein gene SNCA [82], and the vasopressin AVP gene, while DNA hypomethylation was reported in the promoter region of the atrial natriuretic peptide gene ANP [83]. Overall DNA methylation of the MAOA gene promoter has been associated nominally with alcohol dependence as well as nicotine dependence in women, but not in men [84]. Alcohol withdrawal has been shown to cause an increase in methylation in CpGs of the promoter region of the NGF gene in the blood of alcohol-dependent patients, indicating that this neuroprotective protein may be increased in these subjects [85]. The DLK1 gene, whose gene product is important for cellular differentiation, has a normally hypermethylated imprinted region called the IG-DMR. In the sperm of subjects with heavy alcohol consumption, the IG-DMR has been found to be hypomethylated [86], indicating a potential transgenerational effects of alcohol. Hence, chronic alcohol may have long-term effects resulting from changes in epigenetic states that may be passed on to subsequent generations.
Conclusion
Emerging epigenetic findings are now focusing attention on changes in chromatin structure that alter gene expression that are caused by the drugs of abuse. These epigenetic factors may alter initial response to a drug, continued response, the development of tolerance leading to addiction, as well as withdrawal and relapse. Emotional stressors and social adversities may cause an initial epi genetic response that alters reward-signaling pathways, predisposing one to a positive response to drug use. With chronic drug abuse and development of addiction, other epigenetic changes may occur that oppose the initial acute drug response, as illustrated by c-fos activity and cocaine in the nucleus accumbens. From the studies presented above, it appears that epigenetic changes found to be in association with drug addiction are primarily indicative of an epigenetic response to substance exposure, rather than a biological predisposition to develop an addiction. However, studies on the DNA methylation changes found in drug addiction suggest a possible role for epigenetics in predisposing an individual to an increased vulnerability for addiction.
None of these drugs of abuse has shown definitive causal associations with epigenetic changes. The statistical associations are just starting to be replicated, which therefore, are showing more than a single coincidence of epigenetic changes with particular abused drugs. A causal relationship might occur in either direction – as a predisposition or risk factor for drug use, or as a result of using the drug. Although human studies have found associations of epigenetic alterations with drug addiction, such as with OPRM1 DNA methylation in heroin addiction, direct causal evidence demonstrating that these changes altered addiction vulnerability or occurred as the result of use of the specific drug of abuse is lacking.
The elucidation of epigenetic changes that occur in the development of drug dependence or during withdrawal extends our understanding of the mechanistic alterations contributing to addiction. Several pharmacological agents, such as the methylation inhibitor azacitidine, the histone deacetylase inhibitor valproic acid, or the selective histone deacetylase inhibitor SAHA could be explored in future preclinical studies on drug addiction. Azacitidine, a pyrimidine nucleoside analog of cytidine, when metabolized and incorporated into DNA, acts as an irreversible inhibitor of DNA methyltransferases, preventing DNA methylation. Azacitidine is approved for the treatment of several subtypes of myelodysplastic syndrome [87]. Valproic acid (Depakote®, Abbott Laboratories, IL, USA), an anticonvulsant, is a histone deacetylase inhibitor [88]. Valproic acid is used in the treatment of bipolar disorder and the prevention of migraine headaches. Perhaps, in the future, drug dependence will be treated through pharmacotherapeutic management of DNA methylation and histone modifications. Further studies on the epigenetics of drug effects and addiction should reveal new strategies for the prevention and treatment of drug addiction.
Future perspective
Epigenetics is rapidly evolving with the discovery of the mechanisms controlling DNA methylation and chromatin remodeling. Addiction is an orphan field compared with many other areas of disease research and benefits from epigenetic developments in other areas of medicine such as oncology. A major challenge for addiction treatment will be the development of pharmacotherapeutics with agents such as the methylation inhibitor azacitidine, the histone deacetylase inhibitor valproic acid, or the selective histone deacetylase inhibitor SAHA. However, a complication is that addictions are diseases that target very specific regions of the brain. Thus, any epigenetic intervention must not only be specific for a particular segment of the DNA, but it will also need to be targeted to a specific brain region. Epigenetic intervention agents must have delivery systems that direct them to the precise brain location where they are needed. Thus, as new agents are developed, from cancer research in particular, these highly specific chromatin-remodeling agents will require specific methods to be neuroanatomically targeted. These pharmacotherapies may involve a combination of new medication-delivery systems with neurosurgical technologies using precise neuroimaging guidance. New medication delivery systems include nanotubes containing the medication. These nanotubes can then be attached to the outside of a neuron-specific receptor ligand, such as a cocaine-like molecule. This cocaine-like molecule will bind to the same brain receptor as cocaine (e.g. dopamine transporter) and be taken into that neuron where the medication inside the nanotube will be released. Thus, epigenetics in addictions will have a therapeutic application in reversing epigenetic brain changes induced by chronic drug abuse. Whether epigenetic changes can also be used diagnostically in children to identify those at high risk for developing addictions is an open question that may require more time to develop.
Executive summary
Addiction in the USA
- Addictions to cocaine, opiates and alcohol occur in over 5% of the US population and have a 50% or greater contribution from genetic inheritance.
- Addiction is a brain disease with gene–environment (drug) interactions in specific brain areas such as the nucleus accumbens, where drug reinforcement has a final common pathway, and epigenetic factors play a significant role. The drug-specific epigenetic changes described below primarily occur in the nucleus accumbens.
Basic concepts of epigenetics
- Epigenetics is the regulation of heritable and reversible changes in gene expression that occur without alterations to DNA sequence.
- Epigenetic factors include DNA methylation at CpG sites and chromatin remodeling through acetylation, methylation and phosphorylation of the four histone proteins around which DNA is wrapped.
Epigenetic alterations caused by drugs of abuse & addiction
- Cocaine
- – The epigenetics of cocaine addiction includes the action of immediate early gene products, such as c-fos, and more sustained, later accumulation of ΔFosB in the nucleus accumbens through decreases in histone deacetylation.
- – During the development of cocaine addiction levels of other important brain proteins such as Bdnf and Cdk5 also increase in the nucleus accumbens, but Bdnf levels continue to be elevated during withdrawal, while Cdk5 levels drop.
- – Chronic cocaine administration decreases HDAC5 activity, which heightens histone acetylation and transcription in the nucleus accumbens.
- – Higher rates of DNA methylation and upregulated expression of DNA methyltransferases were found in offspring of cocaine-exposed mothers.
- ▪ Opioids
- – Increased OPRM1 promoter methylation was found to be associated in vitro with decreased OPRM1 mRNA content.
- – Altered OPRM1 DNA methylation occurred in leukocytes from former heroin addicts in methadone pharmacotherapy.
- ▪ Alcohol
- – Acute ethanol exposure increases histone 3 and 4 acetylation in specific regions of the amygdala, while withdrawal and associated anxiety decreases histone acetylation. Withdrawal also increases CpG methylation of the promoter of the NGF gene in blood.
- – Chronic ethanol causes demethylation of CpG islands in the NMDA NR2B gene in the cortical neurons of mice and an increase in NR2B expression.
- – The cortex and cultured neurons of alcoholics exhibit elevated DNA methylation of specific prodynorphin CpG sites.
- – Lymphocytes from alcoholics have DNA hypermethylation in the genes for α-synuclein, homocysteine-induced endoplasmic reticulum protein HERP, monoamine oxidase A, and vasopressin and DNA hypomethylation in the ANP gene.
Conclusion
- ▪ Several pharmacological agents might be examined for addictions, such as the DNA methylation inhibitor azacitidine, the histone deacetylase inhibitor, valproic acid or the selective histone deacetylase inhibitor, SAHA.
Acknowledgments
This work was supported by: NIH/NIDA 5 P50 DA018197-05 (TR Kosten), the Veterans Health Administration, the Adrienne Helis Malvin Medical Research Foundation, and the Toomim Family Fund.